Research Themes
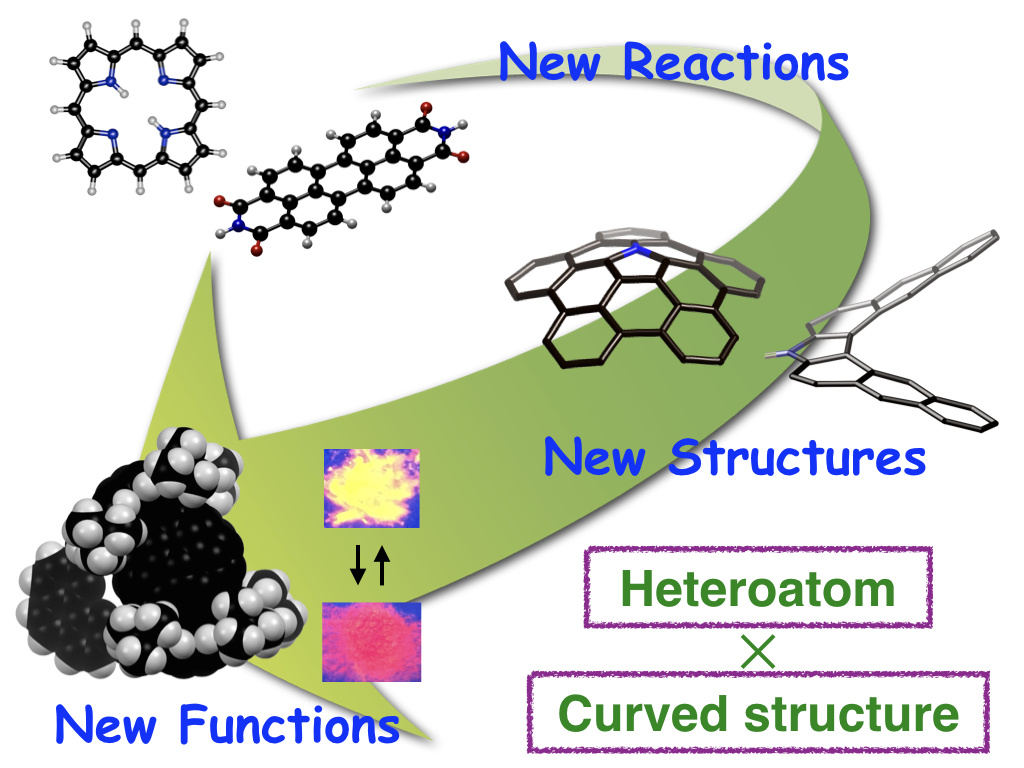
Heteroatom ✕ Curved π-surface
Curved π-conjugated molecules, represented by helicenes and buckybowls, have attracted considerable interest due to their aesthetically pleasing structures. Therefore, they have been investigated in the wide-range of the field of chemistry and material science. However, their synthesis have been mostly performed under high-temperature conditions, that inhibit further development of their chemistry. Although several synthetic chemists have reported a novel synthetic protocol for curved π-conjugated molecules, their chemistry is still in the early developmental stage. Among these, We have investigated synthesis, functionalizations and their material applications in order to disclose their meaning of existence.
In particular, we are now focuing on functions based on combination of heteroatoms with curved π-conjugated structures
Creation of novel curved π-conjugated molecules canteining heteroatoms
Construction of curved structures has required high-energy process such as light-irradication and Flash Vacuum Pyrolysis. On the other hand, we developed novel synthetic protocols for converting a planar π-conjugated molecule into a curved π-conjugated molecules under mild reaction conditions. According to this method, we have succeeded in the synthesis of a variety of heteroatom-containing curved π-conjugated molecules
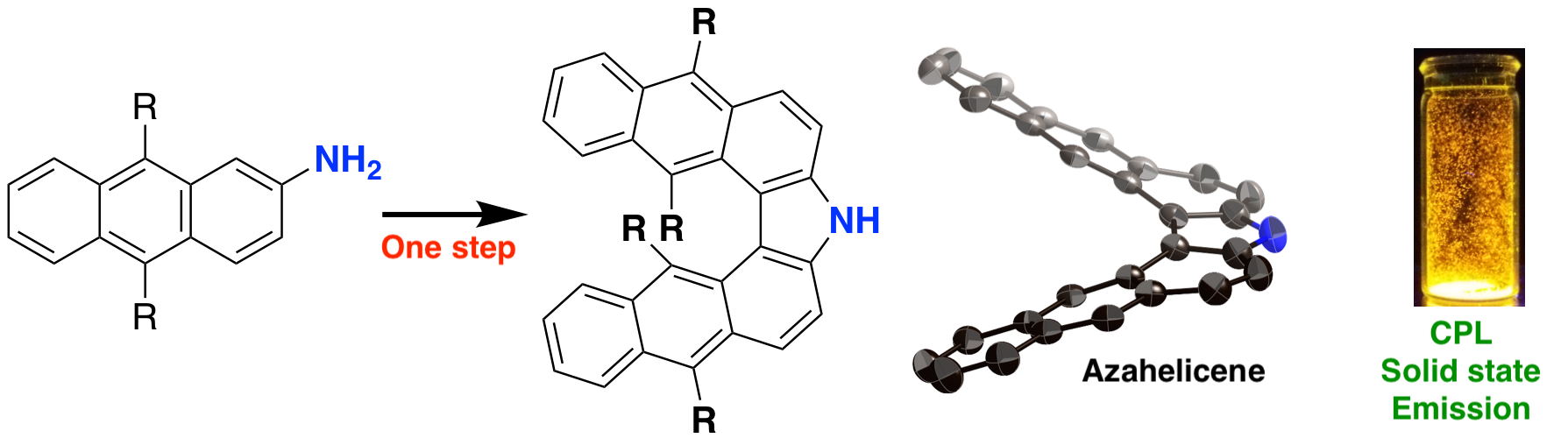
- Synthesis and development of π-conjugated molecules with helical structures
- Helicenes, which have helical skeleton, have been one of the well-investigated curved π-conjugated molecules. Most synthesis of helicenes require light irradiation step. Recently, helicenes with remarkable optocal characteristics have been investigated for efficient chiroptical compounds. Among these, we succeeded in bending oligoacenes with highly emissive feature into π-extended heterohelicenes, which show remarkable emission and circularly polarized luminescence.
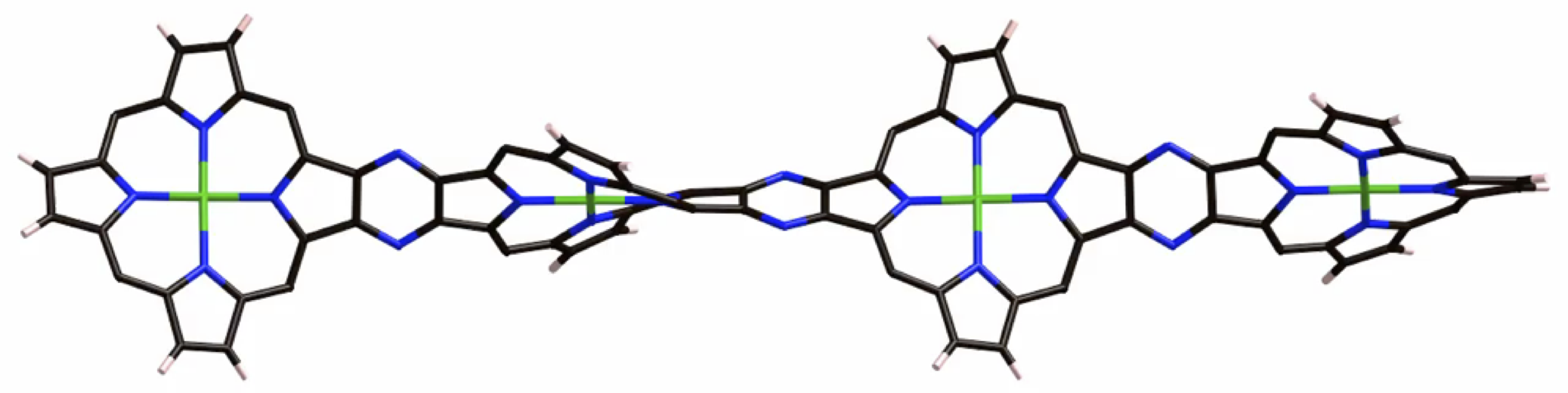
According to this protocol, we also succeeded in the synthesis of twisted porphyrin oligomers with ca. 360° twisting angle. This value is the largest value reported for fused π-conjugated molecules.
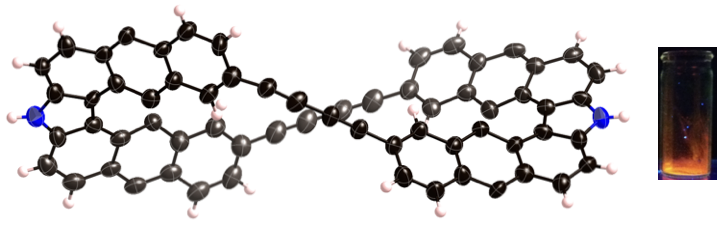
- Synthesis of heteroatom "embedded" buckybowls
-
Buckybowl, i.e. bowl-shaped π-conjugated molecules, have been studied as a key building block for bottom-up synthesis for fullerenes and varbon nanotubes. Among these studies, we succeeded in the synthesis of nitrogen-emmbedded buckybowl "azabuckybowl" as the first example of heteroatom "embedded" type bowl-shaped π-conjugated molecules.
→ Highlighted by Natureaia.com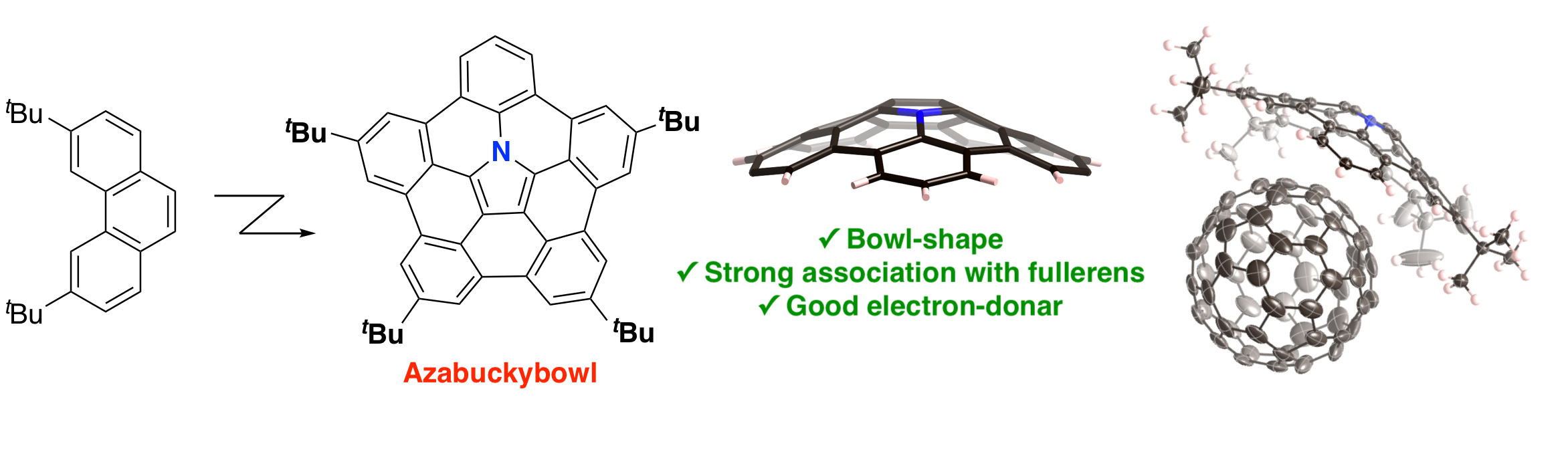
Creaction of novel fucntions by combination of heteroatoms with curved-π structures
- Development of the "Chemistry of Heterobuckybowl"
- Azabuckybowl possesses both nature of nitrogen atom and characteristics due to curved–π structure, and show strong interaction with fullerenes. Utilizing these properties, we developed supramolecular system based on azabuckybowl such as supramolecular polymer and host-guest complex with two-photon absorption cross-section. These are promising properties for the application to nanomaterials such as three-dimensional memory and photodynamic therapy.
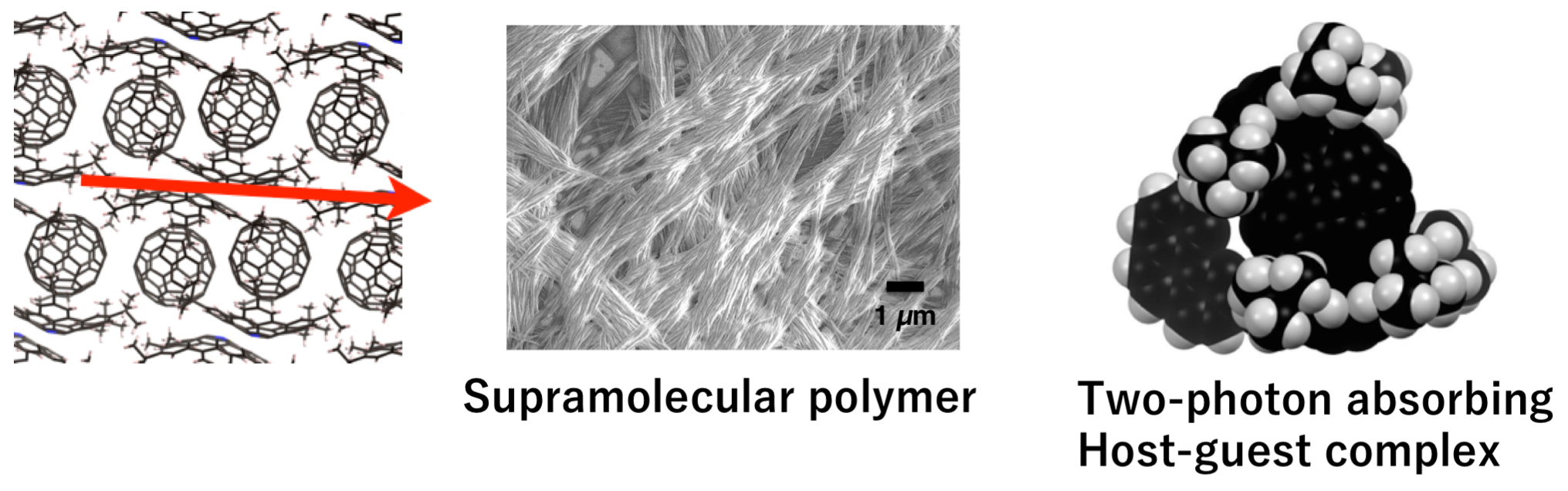
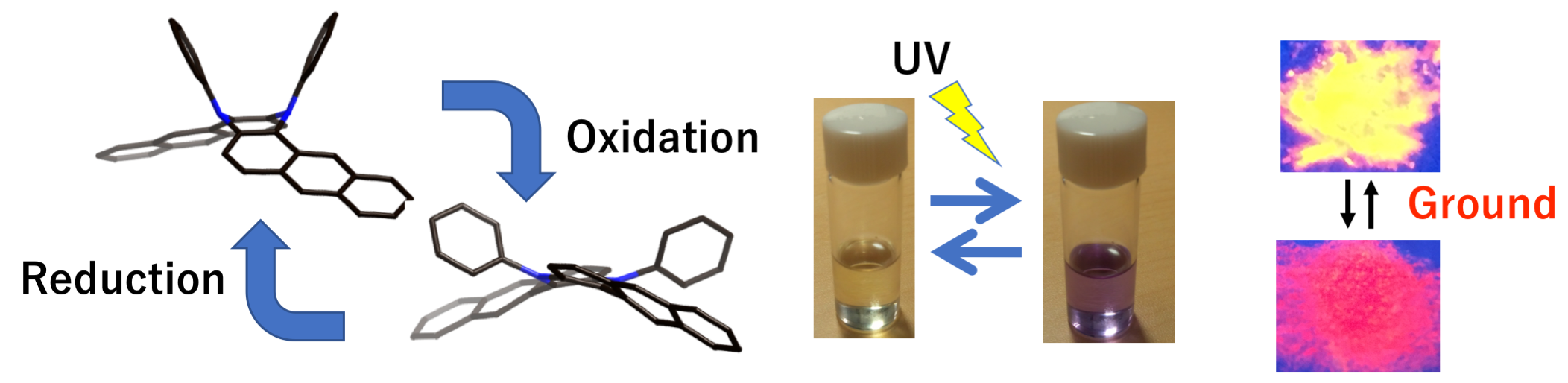
- Development of stimuli-responsive three-dimensional π-conjugated molecules
- Moleucles with responsiveness by external-stimuli have been widely investigated as potential candidates for the applications to sensors, switching devaices and so on. We produced three-dimensional π-conjugated molecules, whose color, emission and conformation were changed upon external stimuli such as light irradiation, electrochemical oxidation, and mechanical stimuli.